Open Doctoral Positions 2023
With over 50 research groups from the Faculty of Biology, the Faculty of Medicine, the Veterinary Medicine Faculty, and the Faculty of Chemistry and Pharmacy of Ludwig Maximilian University (LMU) München, the LSM in its prominent location within the HighTechCampus in Martinsried south of Munich contributes to the enormous possibilities for support, interdisciplinarity and constant scientific input from the surrounding laboratories.
Available research projects cover areas from Biochemistry, Biophysics, Cell and Developmental Biology, Ecology, Evolutionary Biology, Genetics, Microbiology, Pharmacology, and Plant Sciences.
The LSM Graduate School overs three different types of financing, which are displayed in the graphic below.
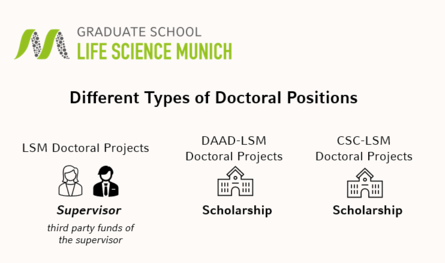
All LSM doctoral projects are financed by third-party funds of the supervisor. Therefore, the main decision on your application will be done by your potential new supervisor.
The DAAD-LSM and CSC-LSM doctoral projects are financed by DAAD and CSC-LMU, respectively. After matching with your potential new supervisor, the final decision on awarding the scholarship is made by the institute.
Have a look at the different types of open positions.
More info about the LSM faculty members´research could be viewed here

